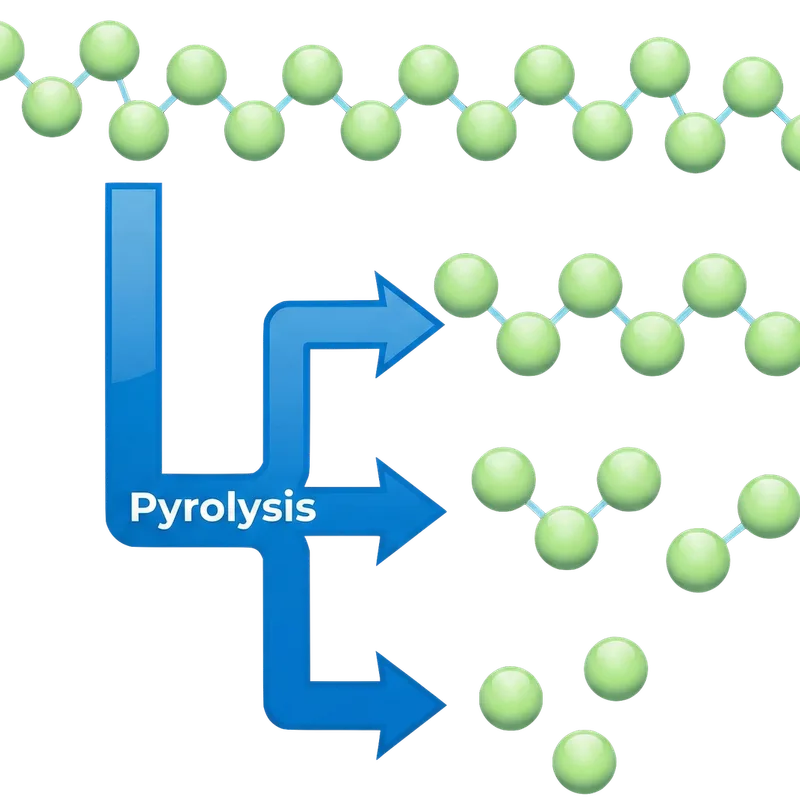
What is Pyrolysis?
From Greek: pyr (πῦρ) = fire + lysis (λύσις) = breaking apart
Waste Plastic
Waste Tyres
Biomass
Pyrolysis Oil
Syngas
Char / Carbon Black
Legend
How Does Pyrolysis Work?
Unlike incineration, pyrolysis operates in an oxygen-free environment. Instead of burning, the feedstock's long-chain molecules are thermally cracked into shorter, commercially valuable products. The process follows five steps:
Feedstock Preparation
Raw material (plastic, tyres, or biomass) is collected, sorted, and shredded to a uniform size. Metals and inert contaminants are removed. For biomass, moisture content is reduced through drying.
Oxygen-Free Heating
The prepared feedstock enters a sealed reactor and is heated to 300-700 °C. Since no oxygen is present, the material does not combust — it thermally decomposes, breaking molecular bonds.
Thermal Decomposition
Long-chain polymers and complex organic molecules crack into shorter chains. This produces a mixture of hot vapours (condensable and non-condensable gases) and a solid carbon-rich residue (char).
Condensation & Separation
Hot vapours pass through a condensation system. Condensable fractions cool into pyrolysis oil (a liquid hydrocarbon mixture). Non-condensable gases remain as syngas. Solid char is collected from the reactor.
Product Collection & Use
Three products are collected: pyrolysis oil (fuel or chemical feedstock), syngas (often recycled to heat the reactor, making the process self-sustaining), and char (used as carbon black, soil amendment, or solid fuel).

A commercial continuous pyrolysis plant designed by APChemi — featuring automated feeding, reactor, condensation, and product collection systems.
Pyrolysis by Feedstock Type
Different feedstock materials have distinct molecular structures, which affect how they break down during pyrolysis and what products they yield:
Plastic Waste
Polymers like polyethylene (PE), polypropylene (PP), and polystyrene (PS) consist of long carbon chains. Pyrolysis breaks these into shorter hydrocarbon chains that form pyrolysis oil, which can be refined into diesel, gasoline, or used as a chemical feedstock. This process is also known as chemical recycling — it can handle mixed and contaminated plastics that mechanical recycling cannot. Learn more about plastic pyrolysis →
Waste Tyres
Tyres contain vulcanised rubber (cross-linked with sulfur bridges), carbon black, and steel wire. Pyrolysis breaks the rubber chains and sulfur cross-links, yielding pyrolysis oil (~40-45%), recovered carbon black (~30-35%), syngas (~10-15%), and recoverable steel wire (~10-15%). Learn more about tire pyrolysis →
Biomass
Wood, agricultural residue, and organic waste consist of cellulose, hemicellulose, and lignin — ring-structured natural polymers with oxygen bridges. Pyrolysis breaks these into bio-oil, syngas, and biochar. Biochar is particularly valuable: when returned to soil, it improves water retention, nutrient availability, and sequesters carbon for hundreds of years. Learn more about biomass pyrolysis →

Wondering which pyrolysis process is right for your feedstock? APChemi offers R&D lab testing to determine optimal conditions and expected yields for your specific material.
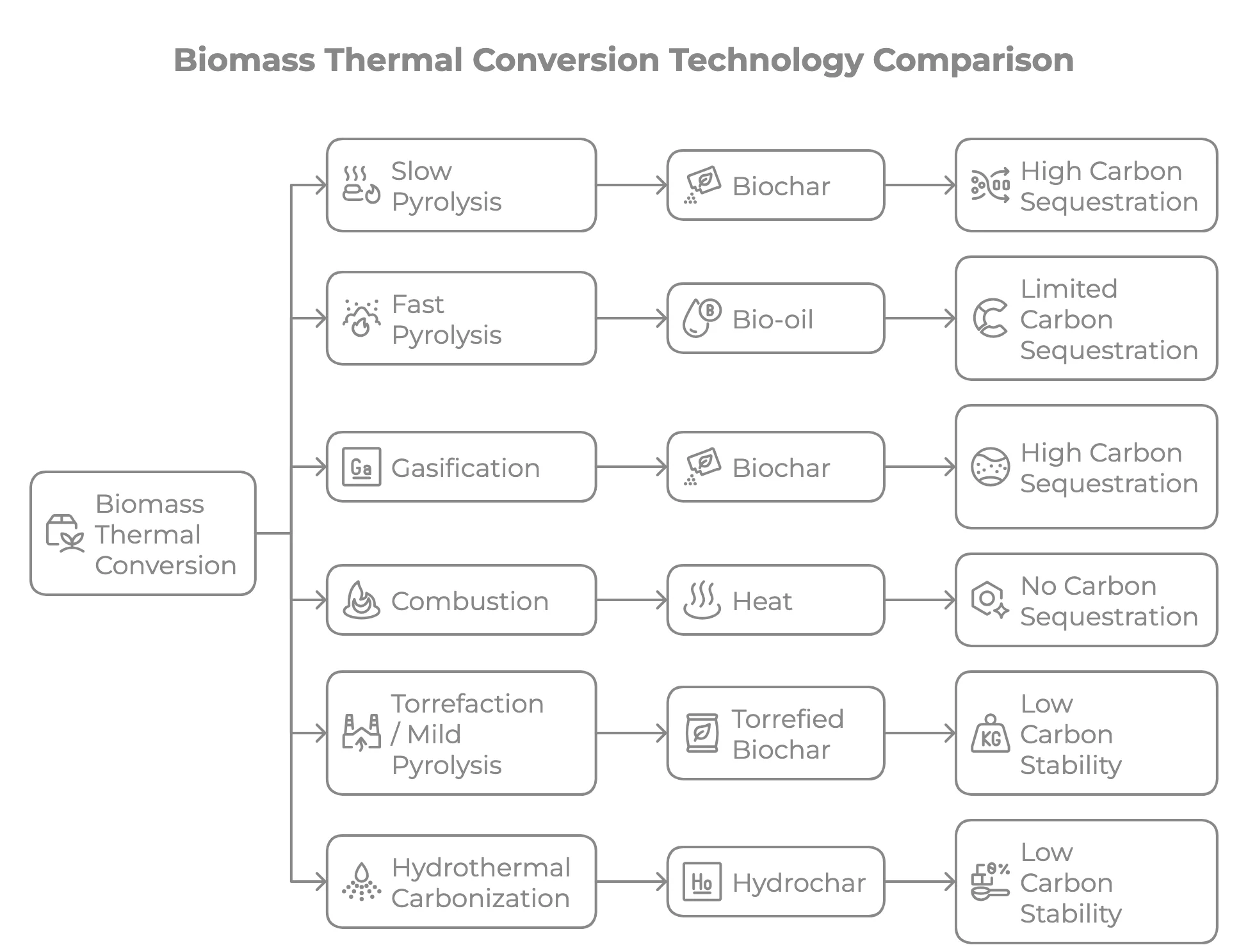
Types of Pyrolysis
Pyrolysis processes are classified by heating rate, temperature, and residence time. These parameters determine the ratio of oil, gas, and char produced:
| Parameter | Slow Pyrolysis | Fast Pyrolysis | Flash Pyrolysis |
|---|---|---|---|
| Temperature | 300-500 °C | 400-600 °C | 500-1,000 °C |
| Heating rate | 0.1-1 °C/s | 10-200 °C/s | >1,000 °C/s |
| Residence time | Minutes to hours | 0.5-2 seconds | <1 second |
| Primary product | Char (35-40%) | Bio-oil (60-75%) | Syngas (60-80%) |
| Oil yield | 20-30% | 60-75% | 10-20% |
| Gas yield | 20-30% | 10-20% | 60-80% |
| Typical use | Charcoal & biochar production | Bio-oil for fuel & chemicals | Syngas & hydrogen production |
| Common reactors | Batch kilns, auger reactors | Fluidised bed, rotating cone | Entrained flow, drop-tube |
For waste plastic and tyre pyrolysis, most commercial plants operate in the slow-to-moderate range (400-550 °C) to maximise oil yield. Fast pyrolysis is more commonly used for biomass-to-bio-oil conversion. See our guides on continuous pyrolysis and batch pyrolysis for more on reactor types.
Pyrolysis vs. Incineration vs. Gasification
All three are thermal waste treatment processes, but they differ fundamentally in oxygen levels, operating conditions, and outputs:
| Parameter | Pyrolysis | Incineration | Gasification |
|---|---|---|---|
| Oxygen | None (oxygen-free) | Excess (oxygen-rich) | Limited (sub-stoichiometric) |
| Temperature | 300-700 °C | 800-1,000 °C | 700-1,300 °C |
| Reaction type | Endothermic (absorbs heat) | Exothermic (releases heat) | Exothermic (partial oxidation) |
| Primary output | Oil, syngas & char | Heat, CO₂ & ash | Syngas (CO + H₂) |
| Energy recovery | Stored in oil & gas products | Direct heat / steam | Stored in syngas |
| Emissions | Minimal (closed system) | CO₂, NOₓ, SOₓ, dioxins | Low (with gas cleanup) |
| Material recovery | High (oil + char reusable) | Low (ash only) | Moderate (syngas) |
| Circular economy fit | Excellent | Poor | Good |
For a deeper dive into gasification, see our pyrolysis vs gasification comparison.
Applications of Pyrolysis
Pyrolysis technology is deployed across multiple industries, converting waste materials into commercially valuable products:
Plastic Recycling
Chemical recycling of mixed, contaminated, or multi-layer plastics that mechanical recycling cannot process — converting waste plastic back into virgin-grade feedstock.
Fuel Production
Pyrolysis oil from waste tyres and plastics can be used as industrial fuel oil or further refined into diesel, gasoline, and marine fuel via distillation.
Agriculture
Biochar from biomass pyrolysis improves soil structure, retains water and nutrients, and sequesters carbon for centuries when applied as a soil amendment.
Chemical Industry
Pyrolysis oil serves as feedstock for producing ethylene, propylene, and other base chemicals — replacing fossil-derived naphtha in petrochemical plants.
Energy & Power
Syngas produced during pyrolysis can fuel industrial boilers, generators, or be converted to hydrogen. Many plants use syngas to power the reactor itself.
Tyre Recycling
End-of-life tyres yield pyrolysis oil, recovered carbon black (rCB) for rubber manufacturing, steel wire for scrap recycling, and syngas for energy.

Pyrolysis oil fractions after distillation — ranging from light naphtha to heavy fuel oil.
Ready to explore pyrolysis for your project? APChemi has designed and delivered 49+ pyrolysis plants across 15+ countries with 12+ patents. Get a free consultation.
Environmental Benefits
Pyrolysis offers significant environmental advantages over traditional waste disposal methods like landfilling and incineration:
Landfill Diversion
Converts waste plastic, tyres, and biomass that would otherwise occupy landfills into reusable products, significantly reducing waste volume.
Lower Emissions than Incineration
Because pyrolysis operates in an oxygen-free, closed system, it produces significantly fewer greenhouse gases, NOₓ, SOₓ, and dioxins compared to incineration.
Carbon Sequestration
Biochar produced from biomass pyrolysis locks carbon in a stable solid form for hundreds to thousands of years. When applied to soil, it actively removes CO₂ from the carbon cycle.
Circular Economy
Pyrolysis enables a closed-loop model — waste materials are converted back into fuels and chemical feedstocks, reducing dependence on virgin fossil resources.
Energy Self-Sufficiency
The syngas produced during pyrolysis can be recycled to heat the reactor, making many commercial pyrolysis plants energy self-sufficient after initial start-up.
Reduced Fossil Fuel Dependence
Each barrel of pyrolysis oil displaces a barrel of crude oil. Pyrolysis-derived fuels are 14-39% less carbon-intensive than traditionally refined petroleum products.
For a comprehensive analysis of the environmental impact of pyrolysis technology, including carbon credit pathways and lifecycle assessment data, see our environmental impact guide.

Biochar produced from biomass pyrolysis — a stable form of carbon that sequesters CO₂ for centuries when applied to soil.
Frequently Asked Questions
Pyrolysis is the thermal decomposition of organic materials at elevated temperatures (300-700 °C) in the absence of oxygen. It breaks down long-chain molecules into three commercially valuable products: pyrolysis oil (40-65% yield), syngas (10-25% yield), and char/biochar (15-40% yield). Unlike incineration, pyrolysis operates in a closed, oxygen-free system with minimal emissions.
There are three main types: Slow pyrolysis (300-500 °C, produces mainly char/biochar at 35-40% yield — ideal for carbon removal), Fast pyrolysis (400-600 °C, maximises bio-oil at 60-75% yield), and Flash pyrolysis (500-1,000 °C, maximises syngas at 60-80% yield). Most commercial plastic and tire pyrolysis plants operate in the slow-to-moderate range (400-550 °C) to maximise oil yield.
Pyrolysis operates in an oxygen-free environment at 300-700 °C and produces reusable products (oil, gas, char). Incineration burns waste with excess oxygen at 800-1,000 °C, producing mainly heat, CO2, and ash. Pyrolysis has minimal emissions and fits a circular economy model, while incineration generates significant greenhouse gases, NOx, SOx, and dioxins. Learn more about the environmental benefits.
Pyrolysis produces three products: Pyrolysis oil (40-65% yield) — used as fuel or refined into diesel and chemical feedstock via distillation; Syngas (10-25% yield) — used for heating the reactor itself or for hydrogen production; and Char/Carbon black (15-40% yield) — used as solid fuel, biochar for soil amendment and carbon sequestration, or recovered carbon black for rubber manufacturing.
Common feedstocks include waste plastics (PE, PP, PS — yielding 60-80% oil), end-of-life tyres (yielding oil, recovered carbon black, steel, and gas), and biomass (wood, agricultural residue, organic waste — yielding bio-oil, syngas, and biochar). Each feedstock type requires specific reactor design and operating parameters. APChemi offers R&D lab testing to determine optimal conditions for your specific feedstock.
Pyrolysis plant costs range from $50,000 for small batch units to $10M+ for large continuous commercial plants. A mid-range continuous plant (10-30 TPD) typically costs $200K-$3M for equipment, with total installed cost 1.5-2.5x that amount. ROI timelines range from 2-5 years depending on feedstock, products, and local market prices. See our detailed cost guide and ROI calculator.
Get a Free Consultation
Tell us about your pyrolysis project and our engineers will get back to you within 24 hours.
Explore More
Biomass Pyrolysis Plant
How pyrolysis converts biomass into biochar, bio-oil, and syngas.
Tire Pyrolysis Plant
Pyrolysis of end-of-life tires into oil, carbon black, and steel.
Plastic Pyrolysis Plant
Chemical recycling of waste plastic via pyrolysis.
Pyrolysis Plant Cost Guide
Comprehensive pricing for batch, continuous, and turnkey plants.
Chemical Recycling
How pyrolysis enables plastic-to-plastic circular economy.
Biochar & Carbon Removal
Using biomass pyrolysis for permanent carbon dioxide removal.